Stéphane Léon et al in Nature Communications
In this paper, the authors have explored the complex relationship between obesity and the hypothalamic nervous system, a critical regulator of feeding behaviour and metabolic health. The hypothalamus contains a remarkable diversity of neurons that orchestrate behavioural and metabolic outputs in a highly plastic manner. Neuronal diversity is key to the hypothalamic functions that allow rapid and precise monitoring of the body’s needs and subsequent adjustments via cross-talk mechanisms with peripheral blood-borne signals.
Contrary to the traditional view that neural diversity in the brain region is fixed during embryonic development, the evidence presented challenges this notion.
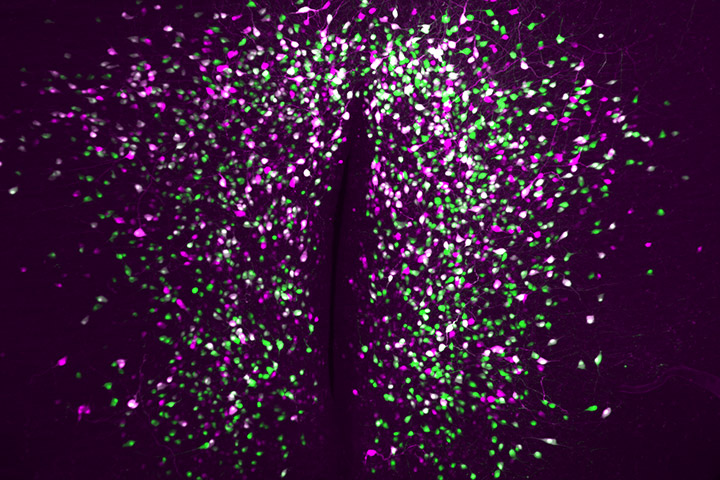
Using state-of-the-art lineage tracing and single cell profiling of hypothalamic pre-opiomelanocortin (Pomc) expressing neurons in adult mice, the researchers have uncovered special ‘ghost’ neurons, characterised by minimal expression of both the main identity marker Pomc and several other markers that define this neuronal population.
Unlike their classical Pomc counterparts, ghost neurons have distinct molecular properties that make them ‘invisible’ (hence ‘ghosts’) to available neuroanatomical approaches and promoter-based reporter mice for studying Pomc biology. These atypical cells also have distinct and quite different functional properties and spatial localisation compared to classical Pomc neurons. Interestingly, the number of atypical ghost neurons increases in diet-induced obese mice, independent of neurogenesis or cell death, suggesting a remarkable adaptability of neuronal identities in response to adult-onset obesogenic stimuli.
This study not only illuminates the remarkable adaptability of hypothalamic neurons but also underscores the potential impact of changes in neuronal identity maintenance throughout adulthood on the development or progression of obesity. This tantalising hypothesis, which is ripe for further exploration, holds promise for the discovery of molecular neuronal targets that could be used to tackle the pervasive health challenge of obesity.
Reference
Single cell tracing of Pomc neurons reveals recruitment of ‘Ghost’ subtypes with atypical identity in a mouse model of obesity
Stéphane Leon 1 , Vincent Simon 1 , Thomas H Lee 1 , Lukas Steuernagel 2 , Samantha Clark 1 , Nasim Biglari 2 , Thierry Lesté-Lasserre 1 , Nathalie Dupuy 1 , Astrid Cannich 1 , Luigi Bellocchio 1 , Philippe Zizzari 1 , Camille Allard 1 , Delphine Gonzales 1 , Yves Le Feuvre 1 , Emeline Lhuillier 3 , Alexandre Brochard 1 , Jean Charles Nicolas 1 , Jérémie Teillon 4 , Macha Nikolski 5 6 , Giovanni Marsicano 1 , Xavier Fioramonti 7 , Jens C Brüning 2 8 9 10 11 , Daniela Cota 1 , Carmelo Quarta 12
1 University of Bordeaux, INSERM, Neurocentre Magendie, U1215, F-33000, Bordeaux, France.
2 Department of Neuronal Control of Metabolism, Max Planck Institute for Metabolism Research, Cologne, Germany.
3 University of Toulouse III Paul Sabatier, INSERM, Institut des Maladies Métaboliques et Cardiovasculaires, U1297, 31400, France; GeT-Santé, Plateforme Génome et Transcriptome, GenoToul, Toulouse, France.
4 University of Bordeaux, CNRS, INSERM, BIC, US4, UAR 3420, F-33000, Bordeaux, France.
5 University of Bordeaux, Bordeaux Bioinformatics Center, Bordeaux, France.
6 University of Bordeaux, CNRS, IBGC UMR 5095, Bordeaux, France.
7 University of Bordeaux, INRAE, Bordeaux INP, NutriNeuro, UMR 1286, F-33000, Bordeaux, France.
8 Center for Endocrinology, Diabetes and Preventive Medicine (CEDP), University Hospital Cologne, Cologne, Germany.
9 Excellence Cluster on Cellular Stress Responses in Aging Associated Diseases (CECAD) University of Cologne, Cologne, Germany.
10 Center for Molecular Medicine Cologne (CMMC), University of Cologne, Cologne, Germany.
11 National Center for Diabetes Research (DZD), Neuherberg, Germany.
12 University of Bordeaux, INSERM, Neurocentre Magendie, U1215, F-33000, Bordeaux, France. .
DOI: 10.1038/s41467-024-47877-2
Mise à jour: 03/06/24
