Muriel Thoby-Brisson in Molecular Therapy – Nucleic Acids
Identification of a therapeutic molecule alleviating breathing impairments associated with the Central Congenital Hypoventilation Syndrome (CCHS).
Central Congenital Hypoventilation syndrome (CCHS) is a rare genetic disease associated with life-threatening breathing deficiencies. CCHS is caused by mutations (polyalanine expansion for most cases) in the PHOX2B gene, a gene controlling the development of the autonomic nervous system that includes the cardiovascular, digestive circuits and the central respiratory networks. CCHS patients suffer from a strong hypoventilation (abnormally low basal breathing frequency and a high occurrence of apneas) and a loss of central chemoreception (mainly no response to hypercapnia). These respiratory defaults are lethal, no pharmaceutical treatment exists so far and survival absolutely requires burdensome mechanical ventilation. Therefore, identifying molecules with potential alleviating effects is of primary importance for patients.
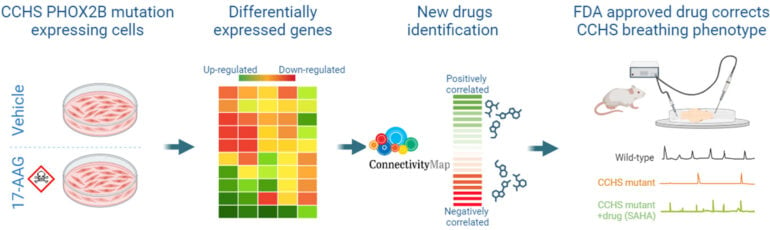
In this study we used cellular and animal models of CCHS to select potential therapeutic molecules restoring PHOX2B function and improving the autonomously-produced central respiratory command. In CCHS, PHOX2B mutations lead to cytoplasmic PHOX2B protein aggregations, thus compromising its transcriptional capability. So, first, on cell culture we investigated the properties of several molecules to treat PHOX2B protein aggregates and to restore its function. We found that Heat Shock Protein inhibitors and Histone Deacetylase inhibitors are the most effective in reducing PHOX2B aggregates and improving its transcriptional activity. Second, mutant mice carrying heterozygous PHOX2B+7Ala expansion of the 20 polyAla tract (Phox2b27Ala/+) recapitulate the hypoventilatory features of CCHS, thus offering a relevant tool for evaluating the abnormal respiratory parameters associated with CCHS. Using isolated brainstem preparations from Phox2b27Ala/+ mice, which contain the main networks constituting the central respiratory command, we established that exposure for several hours to the most promising molecule tested in vitro Vorinostat (also called SAHA) results in a significant compensation for the main respiratory deficits characterizing CCHS. So our work has identified SAHA as a promising drug for the treatment of CCHS.
These exciting results represent a step towards the identification of drugs capable of counteracting the molecular defect and breathing deficiencies found in CCHS, thus paving the way for the development of a pharmacological treatment of this disease. Thus, our findings prove how reversing the hypoventilation phenotype in CCHS patients seems an achievable goal in the coming years.
Reference
Africano C, Bachetti T, Uva P, Pitollat G, Del Zotto G, Giacopelli F, Recchi G, Lenfant N, Madani A, Beckouche N, Thoby-Brisson M*, Ceccherini I*.
Mol Ther Nucleic Acids. 2024 35:102319.
doi: 10.1016/j.omtn.2024.102319. PMID: 39329148
*: co-last authors, co-corresponding authors.
Gabriel Pitollat was a PhD student at INCIA (now post-doc at the Karolinska Institutet, Stockholm, Sweden)
Abstract
Congenital Central Hypoventilation Syndrome (CCHS), a rare genetic disease caused by heterozygous PHOX2B mutations, is characterized by life-threatening breathing deficiencies. The transcription factor PHOX2B is required for the specification of the autonomic nervous system that contains brainstem respiratory control centres. In CCHS, PHOX2B mutations lead to cytoplasmic PHOX2B protein aggregations, thus compromising its transcriptional capability. Currently, the only available treatment for CCHS patients is assisted mechanical ventilation. Therefore, identifying bioactive molecules with potential alleviating effects on CCHS-related breathing impairments is of primary importance. A transcriptomic dataset from transfected cells transiently expressing wild type or mutant PHOX2B was used to query the CMap online tool to identify drug compounds of interest, which were further tested in vitro using fluorescent microscopy and luciferase assay and ex vivo using electrophysiological analysis of fictive respiratory activities on PHOX2B mutant mice isolated brainstem. The histone deacetylase inhibitor SAHA was found to have low toxicity in vitro, to induce relocation of mutated PHOX2B protein in cell nuclei associated with a restoration of proper regulation of PHOX2B target gene expression and to restore respiratory rhythm-related parameters ex vivo. Our results indicate that SAHA is a promising agent for the treatment of breathing deficiencies associated with CCHS.
Mise à jour: 22/11/24
