Three articles of Nora Abrous’ team in Molecular Psychiatry
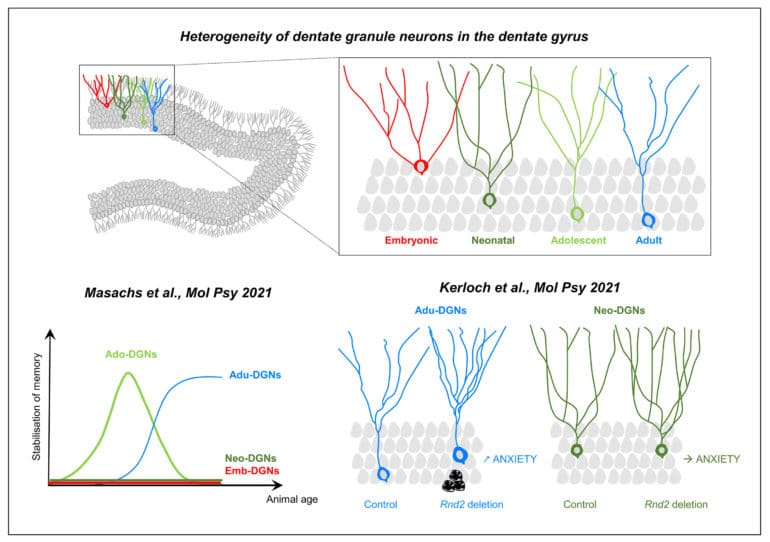
Over the last 30 years, research has firmly established that active neurogenesis continues throughout adult life in discrete areas of the brain, such as in the hippocampal dentate gyrus (DG). This structure presents the peculiarity to exhibit a protracted development: dentate granule neurons (DGNs) are generated from the late embryonic period until adulthood and senescence with a maximal peak during the neonatal period. This continuous addition of DGNs and the resulting heterogeneity of the DG leads us to propose that the temporal origin of DGNs dictates their properties and functions. Supporting this view, we found that adult-born DGNs (Adu-DGNs) and DGNs born during adolescence (Ado-DGNs) are required for spatial learning but at different periods of animals’ life, whereas DGNs born during embryogenesis or early postnatally are not involved (Masachs et al., 2021).
The finding that new neurons are generated throughout life, humans included, has significant implications for brain repair. This has led to tremendous efforts to characterize how new neurons differentiate and integrate into adult neural circuitry. In this view, we have found a critical role for the atypical Rho GTPase Rnd2 in the survival, maturation, and function of adult-born DGNs (Kerloch et al., 2021). Our data has also revealed that Rnd2 has distinct functions during adult and developmental neurogenesis in the DG, arguing against the view that adult neurogenesis is a simple continuation of development.
Based on these observations and our previous work, we have proposed that developmentally-born DGNs establish and maintain a repertoire of basic and rigid behaviors. In contrast, adult-born DGNs sustained flexible behaviors to adapt to environmental changes (Abrous et al., 2021).
The temporal origin of dentate granule neurons dictates their role in spatial memory. Masachs N, Charrier V, Farrugia F, Lemaire V, Blin N, Mazier W, Tronel S, Montaron MF, Ge S, Marsicano G, Cota D, Deroche-Gamonet V, Herry C, Abrous DN. Mol Psychiatry. 2021 Sep 15.
doi: 10.1038/s41380-021-01276-x. Online ahead of print. PMID: 34526669
The dentate gyrus is one of the only brain regions that continues its development after birth in rodents. Adolescence is a very sensitive period during which cognitive competences are programmed. We investigated the role of dentate granule neurons (DGNs) born during adolescence in spatial memory and compared them with those generated earlier in life (in embryos or neonates) or during adulthood by combining functional imaging, retroviral and optogenetic tools to tag and silence DGNs. By imaging DGNs expressing Zif268, a proxy for neuronal activity, we found that neurons generated in adolescent rats (and not embryos or neonates) are transiently involved in spatial memory processing. In contrast, adult-generated DGNs are recruited at a later time point when animals are older. A causal relationship between the temporal origin of DGNs and spatial memory was confirmed by silencing DGNs in behaving animals. Our results demonstrate that the emergence of spatial memory depends on neurons born during adolescence, a function later assumed by neurons generated during adulthood.
The atypical Rho GTPase Rnd2 is critical for dentate granule neuron development and anxiety-like behavior during adult but not neonatal neurogenesis. Kerloch T, Farrugia F, Bouit L, Maître M, Terral G, Koehl M, Mortessagne P, Heng JI, Blanchard M, Doat H, Leste-Lasserre T, Goron A, Gonzales D, Perrais D, Guillemot F, Abrous DN, Pacary E. Mol Psychiatry. 2021 Sep 24.
doi: 10.1038/s41380-021-01301-z. Online ahead of print. PMID: 34561615
Despite the central role of Rho GTPases in neuronal development, their functions in adult hippocampal neurogenesis remain poorly explored. Here, by using a retrovirus-based loss-of-function approach in vivo, we show that the atypical Rho GTPase Rnd2 is crucial for the survival, positioning, somatodendritic morphogenesis and functional maturation of adult-born dentate granule neurons. Interestingly, most of these functions are specific to granule neurons generated during adulthood since the deletion of Rnd2 in neonatally-born granule neurons only affects dendritogenesis. In addition, suppression of Rnd2 in adult-born dentate granule neurons increases anxiety-like behaviour whereas its deletion in pups has no such effect, a finding supporting the adult neurogenesis hypothesis of anxiety disorders. Thus, our results are in line with the view that adult neurogenesis is not a simple continuation of earlier processes from development, and establish a causal relationship between Rnd2 expression and anxiety.
A Baldwin interpretation of adult hippocampal neurogenesis: from functional relevance to physiopathology. Abrous DN, Koehl M, Lemoine M. Mol Psychiatry. 2021 Jun 8.
doi: 10.1038/s41380-021-01172-4. Online ahead of print. PMID: 34103674 Review.
Hippocampal adult neurogenesis has been associated to many cognitive, emotional, and behavioral functions and dysfunctions, and its status as a selected effect or an “appendix of the brain” has been debated. In this review, we propose to understand hippocampal neurogenesis as the process underlying the “Baldwin effect”, a particular situation in evolution where fitness does not rely on the natural selection of genetic traits, but on “ontogenetic adaptation” to a changing environment. This supports the view that a strong distinction between developmental and adult hippocampal neurogenesis is made. We propose that their functions are the constitution and the lifelong adaptation, respectively, of a basic repertoire of cognitive and emotional behaviors. This lifelong adaptation occurs through new forms of binding, i.e., association or dissociation of more basic elements. This distinction further suggests that a difference is made between developmental vulnerability (or resilience), stemming from dysfunctional (or highly functional) developmental hippocampal neurogenesis, and adult vulnerability (or resilience), stemming from dysfunctional (or highly functional) adult hippocampal neurogenesis. According to this hypothesis, developmental and adult vulnerability are distinct risk factors for various mental disorders in adults. This framework suggests new avenues for research on hippocampal neurogenesis and its implication in mental disorders.
Last update 19/10/21