GluK2 Is a Target for Gene Therapy in Drug-Resistant Temporal Lobe Epilepsy
The teams of Christophe Mulle and Valérie Crépel have demonstrated that localized injection of a viral vector targeting GluK2/GluK5 kainate receptors in the hippocampus is a highly promising gene therapy strategy for controlling seizure onset in drug-resistant epilepsy patients. Based on the gene therapy strategy described in the Annals of Neurology article, the Dutch company uniQure, which has acquired the start-up Corlieve Therapeutics – of which Valérie Crépel and Christophe Mulle were scientific co-founders – will launch a Phase I/IIa clinical trial in the USA to for the treatment of drug-resistant temporal lobe epilepsy.
This article is the fruit of a long-standing collaboration between Christophe Mulle (IINS, CNRS, University of Bordeaux) and Valérie Crépel (INMED, Inserm, Marseille). It represents a key step in the development of a gene therapy approach for the treatment of temporal lobe epilepsy. Epilepsy, which consists in the synchronized, abnormal excitation of a group of neurons in the cerebral cortex, affects around 600,000 people in France. Temporal lobe epilepsy is the most common form of epilepsy in adults, with the affected area mainly in the hippocampus. Medical treatment, using antiepileptic drugs to normalize the hyperactivity of cortical circuits, is effective in 70% of cases. For drug-resistant forms, surgical resection is often the only option left. Against this backdrop, the two teams have developed a translational project targeting kainate-type glutamate receptors.
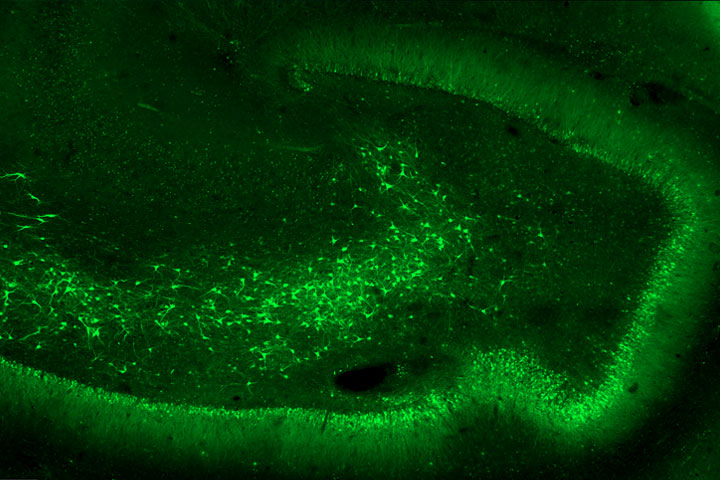
This project is based on the hypothesis that GluK2/GluK5-type kainate receptors localized ectopically at aberrant synapses formed by the sprouting of axons from dentate gyrus granule cells, act as detonators in triggering epileptic discharges in the hippocampus. In 2014, an initial study by these researchers demonstrated that the genetic deletion of grik2, the gene encoding the GluK2 subunit of the kainate-type glutamate receptors, or pharmacological inhibition of GluK2/GluK5 receptors, led to a reduction in the number of spontaneous and recurrent seizures observed in a mouse model of temporal lobe epilepsy. A patent had been filed indicating GluK2/GluK5 as a potential target for treating temporal lobe epilepsy.

In the “Annals of Neurology” article, the teams of Valérie Crépel and Christophe Mulle (and in his team the work carried out mainly by Séverine Deforges, IR CNRS), chose to develop a strategy based on interfering RNAs to reduce grik2 gene expression locally in the hippocampus. The article describes the strategy for selecting interfering RNA sequences in cell models, and the production of a viral vector that enables expression of an anti-grik2 microRNA in hippocampal neurons. This viral vector was injected in vivo into epileptic mice. The article demonstrates the efficacy of this viral vector in decreasing grik2 expression and GluK2 levels in the hippocampus, and ultimately in reducing epileptic activity in mice in vivo. Moreover, administration of the viral vector to organotypic hippocampal slices obtained from surgically resected epileptic patients, led to the suppression of epileptiform discharges recorded in these slices.
In conclusion, localized injection of a viral vector targeting grik2 expression in the hippocampus represents a highly promising gene therapy strategy for controlling seizures in drug-resistant epilepsy patients.
The work described in this article goes hand in hand with an industrial drug development strategy that Valérie Crépel and Christophe Mulle have been pursuing for several years, with the support of Aquitaine Science Transfert, Inserm Transfer and the Kurma Partners investment fund. Valérie Crépel and Christophe Mulle were the scientific co-founders of start-up Corlieve Therapeutics in October 2019, a company that was acquired in July 2021 by uniQure, a Dutch company specializing in the development of gene therapy for neurological diseases.
In September 2023, in parallel with the publication of the article in Annals of Neurology, uniQure issued a press release (see attached pdf), announcing that “the U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug (IND) application for AMT-260, the Company’s gene therapy candidate for refractory mesial temporal lobe epilepsy (MTLE).” “AMT-260 comprises an AAV9 vector that locally delivers two engineered miRNAs designed to degrade the GRIK2 gene and suppress the aberrant expression of glutamate receptor subtype GLUK2 that is believed to trigger seizures in patients with refractory MTLE.”. Accordingly, following the gene therapy strategy described in the Annals of Neurology article, uniQure will launch a Phase I/IIa human clinical trial to be conducted in the US in early 2024.
Article
GluK2 Is a Target for Gene Therapy in Drug-Resistant Temporal Lobe Epilepsy
Céline Boileau*, Séverine Deforges*, Angélique Peret*, Didier Scavarda, Fabrice Bartolomei, April Giles, Nicolas Partouche, Justine Gautron, Julio Viotti, Haley Janowitz, Guillaume Penchet, Cécile Marchal, Stanislas Lagarde, Agnès Trebuchon, Nathalie Villeneuve, Julie Rumi, Thomas Marissal, Roustem Khazipov, Ilgam Khalilov, Fanny Martineau, Marine Maréchal, Anne Lepine, Mathieu Milh, Dominique Figarella-Branger, Etienne Dougy, Soutsakhone Tong, Romain Appay, Stéphane Baudouin, Andrew Mercer, Jared B. Smith, Olivier Danos, Richard Porter, Christophe Mulle#, Valérie Crépel#
2023. Annals of Neurology
doi:10.1002/ana.26723
*co-first authors, # co-senior authors
UniQure’s press release
Donwload the press release (pdf)
Last update 25/10/23
