Fabien Ducrocq, Pierre Trifilieff et al in Cell Metabolism

Psychiatric conditions such as major depression disorder (MDD), bipolar disorders (BP) or schizophrenia (SCZ) are multifactorial diseases caused by both genetic and environmental factors and with a strong developmental etiology. Because they are characterized by distinct diagnostic criteria, these diseases are often considered clinically different. However, a growing body of evidence suggests the existence of a relationship for SCZ, BP and MDD as several common genetic variants, pattern of gene expression as well as environmental factors have been identified as risk factors for their etiology. Moreover, in line with the recent move towards using Research Domain Criteria (RDoC) to describe disorders in terms of observable behaviors rather than using specific diagnoses, they present common symptoms that share similar etiology and neurobiological substrates. Specifically, reward-processing impairment belong to a common symptomatic frame that correlates with a similar pathogenic mechanism: a dysregulation of the mesolimbic system.
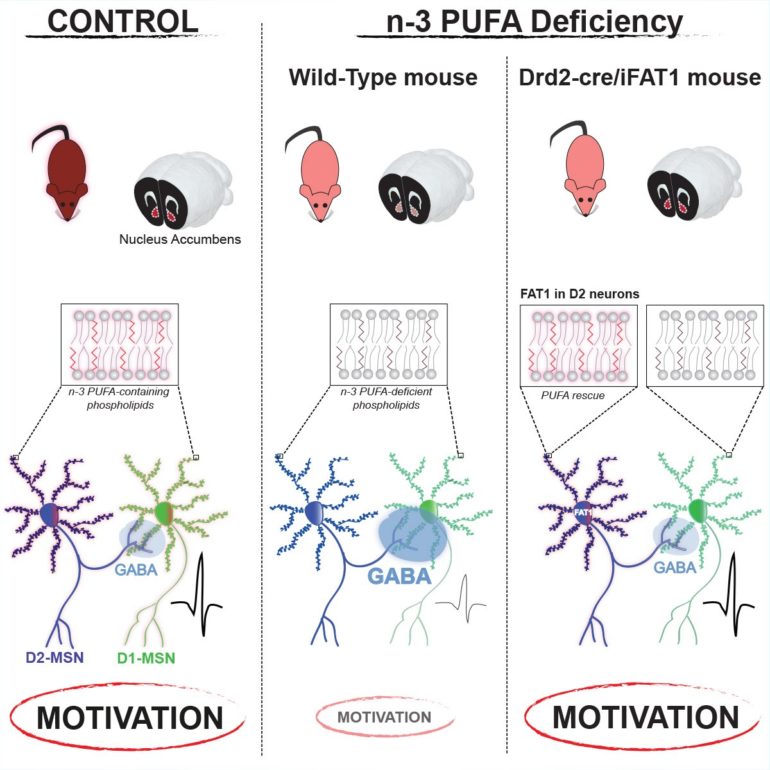
In this context, consistent clinical findings reveal that a shared endophenotype of MDD, BP and SCZ is an alteration in lipid metabolism, and polyunsaturated fatty acids (PUFA) in particular. PUFAs are major components of cell membrane phospholipids where they can modulate membrane properties, act as second messengers and impact the activity of transmembrane and membrane-associated proteins. Herein, we asked if the decrease in n-3 PUFAs, consistently described in these pathologies, could underlie reward-processing deficits. We show that n-3 PUFA deficiency from gestation to adulthood leads to selective motivational impairments. At the neurobiological level, this is related to enhanced lateral inhibition of medium-sized spiny neurons expressing the dopamine D2 receptor (D2R-MSN) onto D1 receptor-expressing MSNs in the ventral striatum, due to increased target projections of D2R-MSNs. Using a unique transgenic mouse model, we show that selectively restoring n-3 PUFA levels in D2R-expressing neurons – but not D1R-expressing cells – during the perinatal period is sufficient to prevent both the rewiring of MSN networks and the motivational deficit.
These results constitute the first demonstration of a causal link between a behavioral deficit and n-3 PUFA decrease in a discrete neuronal population. Moreover, this suggests that the lower n-3 PUFA biostatus described in psychopathologies could participate in the etiology of reward-related symptoms. The origin of n-3 PUFA decrease in psychiatric disorders is unknown but could result from polymorphic variability in genes involved in their metabolism. N-3 PUFA biostatus could therefore constitute a relevant biomarker predictive of specific symptomatic dimensions for psychiatric disorders.
Reference.
https://doi.org/10.1016/j.cmet.2020.02.012
Causal Link between n-3 Polyunsaturated Fatty Acid Deficiency and Motivation Deficits
Cell Metab. 2020 Mar 2.
Ducrocq F1, Walle R2, Contini A2, Oummadi A2, Caraballo B2, van der Veldt S2, Boyer ML2, Aby F2, Tolentino-Cortez T3, Helbling JC2, Martine L4, Grégoire S4, Cabaret S4, Vancassel S2, Layé S2, Kang JX5, Fioramonti X2, Berdeaux O4, Barreda-Gómez G3, Masson E4, Ferreira G2, Ma DWL6, Bosch-Bouju C2, De Smedt-Peyrusse V2, Trifilieff P7.
Author information
- 1 Université Bordeaux, INRAE, Bordeaux INP, NutriNeuro, 33000, Bordeaux, France. Electronic address: .
- 2 Université Bordeaux, INRAE, Bordeaux INP, NutriNeuro, 33000, Bordeaux, France.
- 3 Research Department, IMG Pharma Biotech S.L., BIC Bizkaia (612), 48160 Derio, Spain.
- 4 Centre des Sciences du Goût et de l’Alimentation, AgroSup Dijon, CNRS, INRAE, Université Bourgogne Franche-Comté, 21000 Dijon, France.
- 5 Laboratory for Lipid Medicine and Technology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
- 6 Department of Human Health and Nutritional Sciences, University of Guelph, 50 Stone Road E., Guelph, ON N1G2W1, Canada.
- 7 Université Bordeaux, INRAE, Bordeaux INP, NutriNeuro, 33000, Bordeaux, France. Electronic address: .
Last update 10/03/20